Specification of dendrite identity

My team tries to understand the processes that shape and organize neurons. How are dendrites and axons formed? And how are cargo proteins selectively delivered into these for proper functioning? For this we use a combination of C. elegans genetics, biochemistry and state-of-the-art microscopy. We are part of the Cell Biology, Neurobiology and Biophysics division of the Utrecht University, which also hosts the Biology Imaging Center that will be instrumental for this project. Moreover, we collaborate with the Developmental Biology division and make use of their C. elegans infrastructure.
Learn more on our team's website here.
Contact Martin HARTERINK at m.harterink@uu.nl
NERVSPAN project
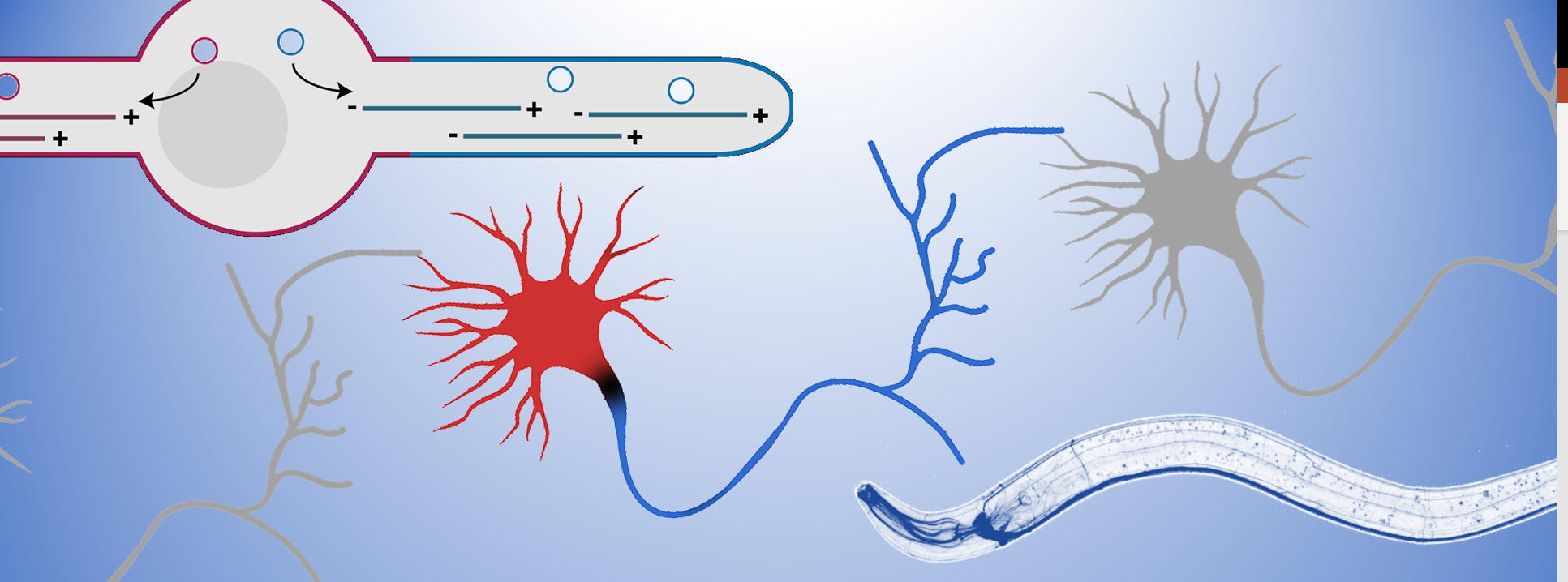
The microtubule cytoskeleton is key to organize neuronal polarity; it provides strength to the cell as well as the “transportation roads” to organize the intracellular environment. Molecular motors of the dynein and kinesin family can walk over the microtubule to either the minus- or plus-end. Since microtubules are organized with their plus-ends outwards in the axon (blue) and minus-ends out in the dendrites (red), this allows for selective transport of cargo into each neurite to set up this axon-dendrite organization. Therefore, my team tries to understand the molecular mechanisms that organize this specific microtubule organization in neurons.
Recently, a pool of microtubule nucleating vesicles were found to localize to the dendrite tip during outgrowth in C. elegans, serving as a microtubule organizing center (MTOC) (Liang et al., 2020). The localization of MTOC-vesicles was found to be critical to generate the dendrite specific minus-end out microtubules and thus to set up axon-dendrite polarity organization. Building on this we investigated 2 microtubule minus-end related proteins in this process; C. elegans NINEIN (NOCA-2) and Camsap (PTRN-1) (He et al., 2022) and found that both have a role in the localization of the MTOC-vesicles. Whereas Camsap proteins are well described microtubule minus-end stabilizing proteins, we found that Ninein localizes to the MTOC-vesicles and to be important for the recruitment of the microtubule nucleating factors. This suggests that microtubule nucleation and stabilization in intimately linked for the proper organization on dendritic microtubules.
It is however still unclear how these MTOC-vesicles get localized to the growing tip and how microtubule nucleation is connected to microtubule stabilization to only maintain the correctly oriented microtubules.
Objectives
Therefore in this project we will investigate:- the extracellular signals that guide the localization of these MTOC-vesicles to the growth dendrite tip;
- the molecular mechanism that translocates these vesicles forward;
- the connection between microtubule nucleation and stabilization to generate minus-end out microtubules.
For this project we will make extensive use of C. elegans genetics (e.g. CRISPR and cell specific gene depletion), biochemistry (protein interaction studies and expansion microscopy) and advanced live-imaging techniques (FRAP and photoactivation microscopy as well as optogenetic controlled protein interaction studies).
Altogether this will help us understand how such special cell as the neuron is able to organize itself with an axon-dendrite organization.