From cell plasticity to regenerative medicine

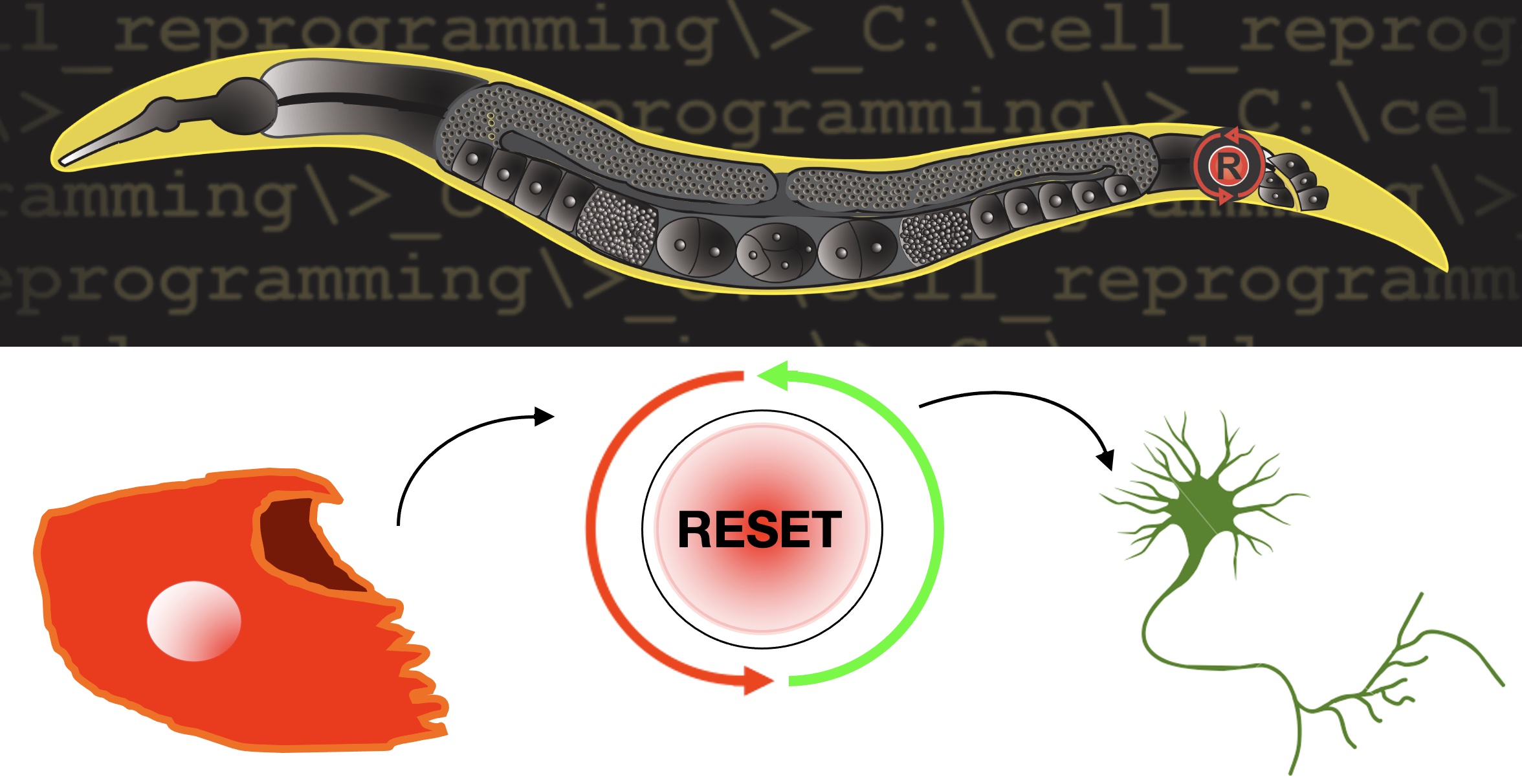
How differentiated cells can change their identity is a fascinating question in biology and has implications for the development of regenerative medicine strategies. Numerous examples of cellular plasticity in physiological, experimental and pathological settings exist, but precisely how a differentiated cell can change its identity remains unknown. Our research tackles this important question by employing a powerful and innovative model, the nematode C. elegans.
Our work has established the worm as a new model to study cellular reprogramming in vivo at the single cell level. Because of the C. elegans invariant cellular lineage, our system gives us access to all the steps of the process, including the early ones, a unique asset. We investigate how specific cells, but not their neighbours, become competent to be reprogrammed. We have begun the systematic identification of the molecular networks and the dissection of the cellular requirements underlying direct cell type conversion in vivo. In addition, we are investigating the following fascinating questions: How can new neurons be generated through reprogramming? What are the cellular steps between 2 identities? Since the differentiated cell identity is usually safely maintained, what are the mechanisms that unlock this maintenance? What is the function of new neurons made by reprogramming and how do they integrate into existing neuronal networks?
To tackle these questions, our research goes from the single cell to an integrated view of the organism in its environment. These integrated approaches will contribute to unravel the key mechanisms that allow a differentiated cell to become plastic and change its identity. Such knowledge has significant therapeutic implications, as it will deepen our understanding of the initiation of certain cancers, and will improve our ability to reprogram cells for regenerative medicine purposes.
You would like to solve the next questions in cellular plasticity? Please apply! Our team is part of the Development & Stem Cells Department, at the IGBMC in Strasbourg, France and has received funding from the INSERM, ANR, ARC and ERC among others. The IGBMC Research Institute provides access to state-of-the-art facilities and a vibrant international research environment, and offers the candidate a unique opportunity to be exposed to multidisciplinary teams and a rich scientific life (seminars, international meetings, scientific clubs, retreats, postdocs&students board, etc).
Learn more on our team's website here.
Contact Sophie Jarriault at sophie@igbmc.fr
NERVSPAN project
Our project aims to understand the steps preceding reprogramming in vivo and the cellular trajectory of a rectal cell that naturally reprograms into a motoneuron. It will use a combination of bioinformatics analysis of the scRNA-seq data and high-end imaging, genetics (including cell-specific gene over-expression or inactivation) and genome editing in vivo (e.g. CRISPR engineering or MosSCI) and for functional validations.
The project is divided into 3 axes:
- Characterisation of the licencing step that allows the reprogramming to start.
- Re-differentiating into a neuron: Transcription Factor waves and cellular steps to determine the dynamic transition states necessary during this original way to make a new neuron.
- Unravel the sex-specific differences while making the same neuron in males and hermaphrodites, and highlight the shared principles between classical embryonic neurogenesis and reprogramming re-differentiation.