Robustness of neuronal excitability and function across gender and lifespan

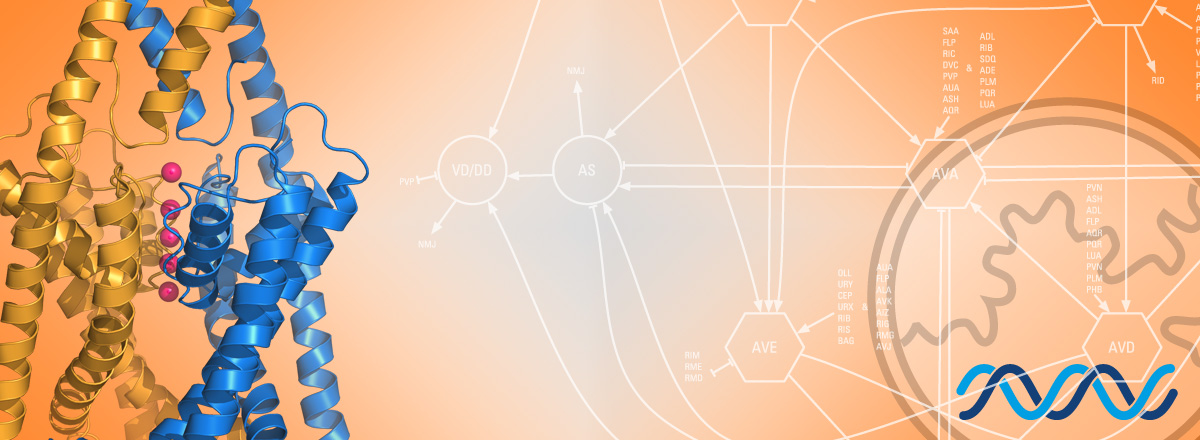
We want to understand how cellular physiology is controled by basic molecular and cellular pathways regulating the biology of potassium-selective ion channels. Recent advances in single-cell transcriptomics have revealed a rich combinatorial code of potassium channels in the nervous system of C. elegans. Combined with the powerful genetic tools available in worms, this knowledge allows us now to directly ask how the electrical identity of neurons is established, how it is maintained, and how it can change with life experience or differ in male/female brains.
As a member of the MeLiS laboratory and the Labex CORTEX initiative, we are embedded in the rich neuroscience environment of Lyon (France). We have ongoing collaborations with national and international partners on basic and applied research topics.
Learn more on our team's website here.
Contact Thomas BOULIN at thomas.boulin@univ-lyon1.fr
NERVSPAN project
Potassium-selective ion channels control neuronal excitability by ensuring neuronal repolarization following an action potential. Single-cell transcriptomic studies in the C. elegans nervous system have revealed that these potassium channels are often expressed in complex combinations of over a dozen channel genes in a single neuron. These different ion channel combinations will determine the biophysical and electrical properties defining neuron-specific functions throughout its life.
Objectives
In this project you will determine:- how this robust electrical phenotype is established at the genetic and cellular level
- how it is maintained or adapted during the worm’s lifetime, and
- whether it differs in neurons shared by males and hermaphrodites.
We will address these questions by integrating transcriptomic and proteomic data, with in situ quantification of protein levels (CRISPR/Cas9 knockins). We will take advantage of behavioral analysis tools developed in this project to probe the functional consequences of observed changes in ion channel repertoires over the lifetime of the worm. The short lifespan of C. elegans provides a unique opportunity to study the evolution of neuronal excitability throughout development, alternative life stages, and ageing. Finally, a fascinating aspect is the sex-specific differences that have emerged from the study of sexually dimorphic neurons that are common to males and hermaphrodites. So far, this has not been addressed through the prism of cellular excitability and ion channel combinatorial codes.
From a conceptual point of view, this project will address for the first time how ion channel degeneracy, variability and covariation are genetically regulated to ensure the resilience and phenotypic stability/plasticity of excitable cells in C. elegans.